Is Your Sleep Routine Aging You? The 5-Point Checklist That Predicts Brain Health (Stop Sleeping Like This)
In the annals of preventative medicine, 2024 and 2025 marked a pivotal shift in how we conceptualize sleep. For decades, sleep was viewed through the lens of immediate performance—fatigue, irritability, and “brain fog” were the accepted costs of deprivation.
However, a landmark study from the Karolinska Institutet, published in eBioMedicine (part of The Lancet Discovery Science), has fundamentally altered this calculus.
By applying machine learning to the MRI scans of thousands of individuals, scientists have successfully quantified the long-term structural cost of poor sleep. The finding is precise and sobering: poor sleep habits can age the brain by approximately 12 months beyond its chronological years.
The Karolinska Protocol: Deconstructing the 12-Month Gap
Phase I: The Wake-Up Call
Summary placeholder.
DREAM DATA:
To fully grasp the significance of the “12-month” statistic, it is necessary to examine the rigorous methodology employed by the researchers. This was not a small-scale observational survey but a massive epidemiological investigation utilizing the UK Biobank, one of the world’s most robust biomedical datasets.
Study Architecture and Methodology
The study analyzed data from 27,500 middle-aged and older adults (aged 40–69 years). Participants underwent T1-weighted magnetic resonance imaging (MRI) of the brain.
The researchers utilized a machine learning algorithm trained on a separate cohort of healthy individuals to recognize the structural signatures of aging. By analyzing over 1,000 distinct brain phenotypes, the algorithm generated a predicted “Brain Age” for each participant.
The difference between this predicted age and the participant’s actual chronological age formed the Brain Age Gap (BAG). A positive BAG indicated accelerated aging, while a negative BAG suggested neuroprotection.
The 5-Point Sleep Composite Score

Crucially, the study did not rely on a single, reductive metric like sleep duration. Recognizing that sleep health is multifaceted, the researchers constructed a composite “healthy sleep score” based on five self-reported factors. This holistic approach captures the complexity of sleep pathology better than duration alone.
Participants were categorized into three tiers based on their aggregate score:
- Healthy Sleep: Score ≥ 4
- Intermediate Sleep: Score 2–3
- Poor Sleep: Score ≤ 1
The Dose-Response Discovery

The analysis revealed a striking linear relationship between sleep quality and brain aging.
The 1-Year Penalty: Individuals in the “Poor Sleep” category exhibited brains that appeared, on average, 1.0 to 1.2 years older than those in the “Healthy Sleep” category.
The Cumulative Effect: For every 1-point decrease in the healthy sleep score, the brain age gap widened by approximately 0.5 years (6 months). This “dose-response” relationship is critical evidence of causality; it suggests that every incremental improvement in sleep habits yields a measurable benefit in preserving brain structure.
Gender Dimorphism: The association between poor sleep and accelerated brain aging was found to be slightly more pronounced in men than in women, though it remained significant for both.
Inflammation as the Biological Mediator

Perhaps the most scientifically significant aspect of the Karolinska study was its investigation into why this aging occurs. The researchers hypothesized that systemic inflammation might be the bridge between poor sleep and brain atrophy.
By analyzing blood biomarkers—specifically C-reactive protein (CRP) and other inflammatory cytokines—they performed a mediation analysis.
The results showed that low-grade systemic inflammation explained approximately 10% of the association between poor sleep and older brain age.
While 10% indicates that inflammation is a partial driver, it also suggests that other mechanisms—likely involving the glymphatic system and oxidative stress—account for the remaining 90% of the effect. This finding places inflammation management at the center of any strategy to mitigate sleep-related brain aging.
The Biology of Decay: Mechanisms of Neurocognitive Aging
The Karolinska study provides the epidemiological evidence, but to intervene effectively, we must understand the cellular and molecular mechanisms at play.
Why does a lack of sleep physically shrink the brain? The answer lies in three interlocking systems: The Glymphatic System, Neuroinflammation, and Mitochondrial Dysfunction.
The Glymphatic System: The Brain’s Night Shift
The most compelling explanation for the link between sleep and brain aging is the glymphatic system. Discovered in 2012 and extensively mapped over the subsequent decade, this system is the brain’s macroscopic waste clearance pathway—essentially, its dedicated plumbing system.
The Mechanism of Clearance: Unlike the peripheral body, the brain lacks a traditional lymphatic system. Instead, it relies on the flow of cerebrospinal fluid (CSF) along perivascular spaces.
This fluid enters the brain tissue, mixes with interstitial fluid (ISF), and washes metabolic waste products toward the venous drainage system.
The Role of AQP4: This process is facilitated by Aquaporin-4 (AQP4) water channels located on the “endfeet” of astrocytes (glial cells) that wrap around blood vessels. These channels act as sluice gates, controlling the flow of fluid.
Sleep Dependency: The critical insight for aging is that the glymphatic system is primarily active during sleep. During wakefulness, norepinephrine (the arousal neurotransmitter) keeps brain cells tightly packed, restricting fluid flow.
During deep, non-REM sleep (Slow-Wave Sleep), the interstitial space expands by up to 60%, allowing CSF to flush through the tissue freely.
The Waste Products: The glymphatic system is responsible for clearing neurotoxic proteins, most notably beta-amyloid and tau—the hallmarks of Alzheimer’s disease. When sleep is fragmented or shortened (as seen in the “Poor Sleep” group of the Karolinska study), this cleaning cycle is interrupted.
The “trash” accumulates, leading to protein aggregation, toxicity, and eventually, the death of neurons. This loss of neuronal volume is what the MRI algorithm detects as “aging”.
The Critical Role of Sleep Position: Research into glymphatic efficiency has uncovered a surprisingly simple variable: body posture.
Studies utilizing dynamic contrast-enhanced MRI and fluorescent tracers in rodent models have demonstrated that the lateral (side-sleeping) position is significantly more efficient for glymphatic clearance than either the supine (back) or prone (stomach) positions.
Physics of Flow: In the lateral position, the alignment of gravity and arterial pulsatility maximizes the kinetic energy available to drive CSF through the brain parenchyma.
Evolutionary Alignment: This finding aligns with the observation that most mammals, and indeed most humans, naturally prefer a lateral sleeping position.
For individuals concerned about brain aging, adopting a lateral sleep posture may be one of the simplest and most effective interventions to enhance nightly “brain cleaning”.
Neuroinflammation and the “Leaky” Barrier
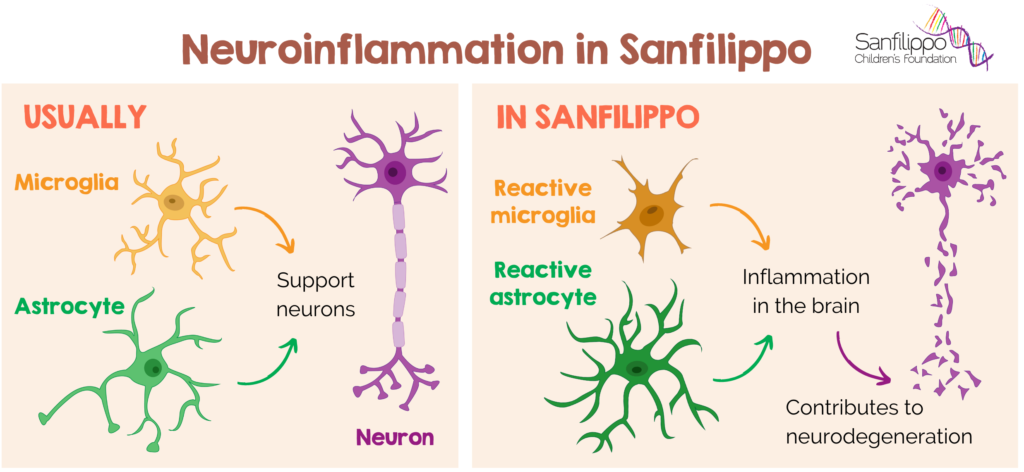
The Karolinska study explicitly identified inflammation as a mediator of brain aging. The mechanism involves the breakdown of the Blood-Brain Barrier (BBB).
The Cytokine Storm: Sleep deprivation triggers a stress response, elevating levels of systemic pro-inflammatory cytokines such as IL-6 and TNF-α.
Barrier Breakdown: Chronic exposure to these inflammatory signals weakens the tight junctions of the BBB, making it permeable or “leaky.” This allows immune cells and inflammatory molecules from the blood to enter the brain’s protected environment.
Microglial Activation: Once inside, these factors activate microglia, the brain’s resident immune cells. In a healthy state, microglia prune weak synapses and support learning.
However, when chronically inflamed, they enter a “primed” or aggressive state, where they may indiscriminately attack healthy synapses and neurons. This process, known as synaptic stripping, leads to a reduction in cortical density—literally, the thinning of the brain’s grey matter.
Mitochondrial Dysfunction and Oxidative Stress
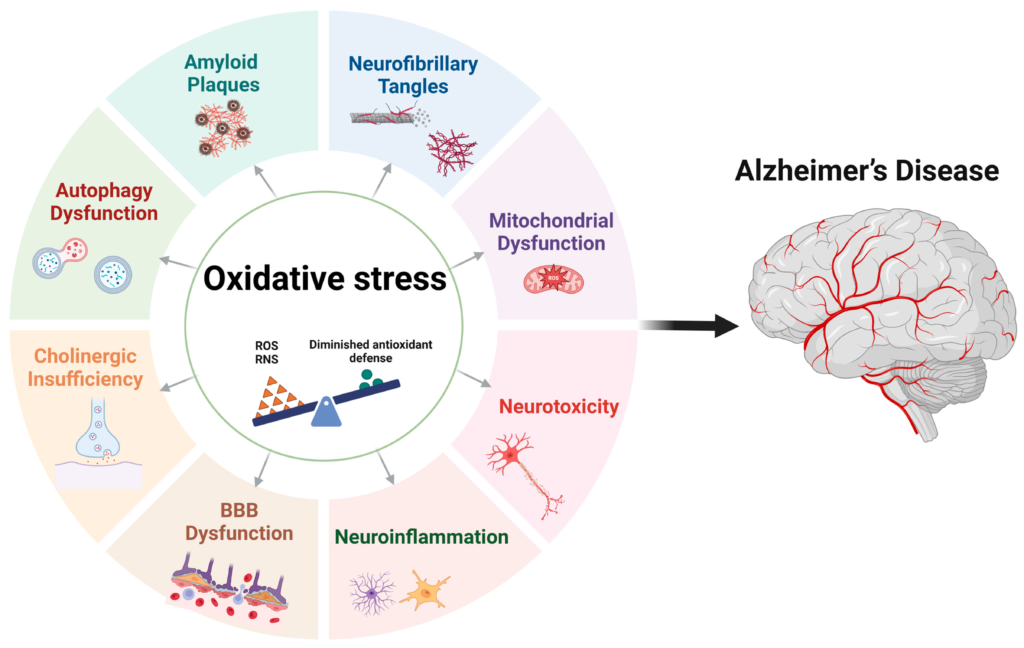
The brain is a metabolic furnace, consuming 20% of the body’s energy despite representing only 2% of its mass. This intense activity generates Reactive Oxygen Species (ROS)—free radicals that damage cellular DNA and proteins.
The Restoration Phase: Sleep is the biological window for mitochondrial repair. During sleep, the production of ATP (energy) is prioritized for cellular maintenance rather than neuronal firing. Antioxidant enzymes are upregulated to neutralize the ROS generated during the day.
The Cost of Wakefulness: When sleep is curtailed, mitochondria are forced to continue energy production without a maintenance break.
This leads to the accumulation of oxidative damage, which accelerates the senescence (aging) of neurons. Over the years, this “oxidative debt” manifests as the structural atrophy measured in the Karolinska study.
The Chronotype Controversy: Larks vs. Owls
One of the 5 factors in the Karolinska “healthy sleep score” was chronotype, with “Morning” preference being scored as healthy and “Evening” preference as unhealthy. This categorization warrants a nuanced discussion, as recent conflicting data have emerged.
The Case Against the Night Owl

Traditional sleep science, supported by the Karolinska findings, suggests that evening types are at higher risk for a range of pathologies.
Behavioral Comorbidities: Research indicates that “Night Owls” are statistically more likely to engage in unhealthy behaviors, including higher rates of smoking, alcohol consumption, and sedentary lifestyles. These behaviors are independent risk factors for brain aging.
Misalignment (Social Jetlag): The primary driver of damage for Night Owls is often social jetlag. An individual biologically wired to sleep from 2:00 AM to 10:00 AM who is forced to wake at 7:00 AM for work is in a state of chronic circadian misalignment.
This misalignment disrupts the hormonal rhythms of cortisol and melatonin, driving the systemic inflammation observed in the Karolinska study.
The Imperial College Counterpoint

However, a major study from Imperial College London, published in mid-2024, presents a conflicting narrative. Analyzing data from 26,000 adults, researchers found that self-declared “Night Owls” generally scored higher on cognitive tests (including memory, reasoning, and processing speed) than “Morning Larks”.
Cognitive vs. Structural: This creates an apparent paradox: Night Owls may have “sharper” cognitive software (Imperial study) while simultaneously accumulating “older” structural hardware (Karolinska study).
The “Mind After Midnight” Hypothesis: Stanford Medicine researchers offer a warning that bridges these views. They found that regardless of chronotype preference, staying awake late into the night (past 1:00 AM) is associated with significantly higher rates of mental health disorders.
Synthesis: The consensus for 2025 is that alignment is king. If an individual is a Night Owl, they must strive to align their lifestyle with their biology to avoid social jetlag.
However, the structural risks identified by Karolinska (inflammation, glymphatic failure) are likely exacerbated by the lifestyle factors often associated with eveningness.
The 2025 Optimization Protocol: Reversing the Damage
The most empowering takeaway from the Karolinska study is the confirmation that brain aging via sleep loss is a modifiable process. The brain is plastic; it responds to changes in input.
By optimizing sleep hygiene, we can reduce inflammation, restore glymphatic flow, and potentially reverse the “Brain Age Gap.”
The following protocol integrates the latest 2025 guidelines, combining behavioral tools, environmental optimization, and evidence-based supplementation.
Pillar 1: Circadian Anchoring (Timing and Light)

To fix sleep quality, one must first fix the wake cycle. The brain’s master clock, the Suprachiasmatic Nucleus (SCN), requires strong environmental cues (zeitgebers) to regulate the sleep-wake rhythm.
Morning Optical Flow: View sunlight outdoors within 30-60 minutes of waking. This stimulates the melanopsin-containing retinal ganglion cells, triggering a cortisol pulse that promotes alertness and sets the timer for melatonin release ~16 hours later.
Protocol: 10 minutes on clear days, 20-30 minutes on overcast days. If dark (winter/shift work), use a 10,000 lux light therapy lamp.
Sunset Signaling: View low-angle solar light in the late afternoon. This signals the SCN to lower retinal sensitivity to light, protecting against the disruptive effects of artificial light later in the evening.
The “Dark Phase”: Melatonin is easily suppressed by blue/green spectrum light. Between 10:00 PM and 4:00 AM, minimize bright overhead lighting. Use dim, amber-hued lamps or install red-shift software (e.g., flux) on devices. Blue-blocking glasses are a valid tool for 2025 sleep hygiene.
Pillar 2: Environmental Optimization (Temperature and Noise)

Thermal Regulation: The body’s core temperature must drop by approximately 2-3°F to initiate and maintain deep sleep. A bedroom temperature of 65°F (18°C) is widely cited as optimal.
The Warm Bath Effect: Taking a warm bath or shower 1-2 hours before bed causes vasodilation (widening of blood vessels) in the hands and feet. This radiates heat away from the core, rapidly lowering body temperature and signaling sleep onset.
Auditory Control: Noise pollution fragments sleep, pulling the brain out of deep, restorative stages. In 2025, the trend has shifted from “White Noise” (static) to “Brown Noise” or “Pink Noise”, which have lower frequencies that are more soothing to the brain.
Pillar 3: Nervous System Regulation (NSDR)
For many “poor sleepers” in the Karolinska study (those with insomnia), the issue is not lack of time, but hyperarousal. The nervous system is stuck in sympathetic (fight/flight) mode.
NSDR (Non-Sleep Deep Rest): Popularized by neurobiologists like Dr. Andrew Huberman, NSDR (often based on Yoga Nidra) is a protocol involving 10-20 minutes of guided body scanning and specific breathing patterns.
Mechanism: NSDR mimics the brain wave patterns of the transition state between wakefulness and sleep (theta waves). It has been shown to replenish dopamine levels and reduce cortisol.
Application: Use NSDR in the afternoon (instead of a nap) to reduce fatigue, or in the middle of the night if you wake up and cannot fall back asleep. It reduces the “sleep anxiety” that often exacerbates insomnia.
Pillar 4: Evidence-Based Supplementation
COMPOUND ANALYSIS
Reduces sleep latency; improves depth.
While behavioral interventions are primary, specific compounds can support sleep architecture without the dependency risks of prescription sedatives. Note: Always consult a healthcare provider.
Anti-Inflammatory Support: Given the Karolinska finding on inflammation, dietary inclusion of Curcumin, Fish Oil (Omega-3), and Tart Cherry Juice (natural melatonin + anti-inflammatory) may provide neuroprotective benefits.
The Future of Sleep Science (2025-2026)
As we look toward the future, several trends are reshaping how we approach sleep and brain health.
Sleep Banking

The concept of “Sleep Banking”—getting extra sleep in the days leading up to a known period of deprivation—is being re-evaluated.
While previously dismissed, recent military and athletic research suggests that banking sleep can create a “reserve” that preserves cognitive function and emotional stability during subsequent loss.
However, it is not a perfect shield against the inflammatory effects identified by Karolinska; it is merely a mitigation strategy.
The Rise of “Orthosomnia.”

With the proliferation of wearables (Oura, Whoop, Apple Watch), a new pathology has emerged: Orthosomnia—the perfectionistic quest for ideal sleep data. Patients may sleep reasonably well but feel anxious because their “Sleep Score” is 75 instead of 90.
This anxiety paradoxically raises cortisol and worsens sleep. The 2025 guidance is to use data for long-term trends (weekly/monthly averages) rather than daily judgment. If the tracker causes anxiety, taking a “data sabbat” is recommended.
AI-Driven Sleep Tech
Technology is moving from passive tracking to active intervention.
Closed-Loop Stimulation: New headbands detect slow-wave sleep in real-time and deliver auditory pulses (pink noise) to deepen the waves, theoretically enhancing glymphatic clearance without requiring more time in bed.
Smart Mattresses: Beds that use AI to adjust temperature and firmness throughout the night to prevent wakeups caused by overheating or pressure points.
Conclusion: Closing the Gap
The finding that poor sleep can age the brain by 12 months is a clarion call for the modern era. It quantifies the hidden cost of the “hustle culture” that views sleep as a luxury.
However, the true power of the Karolinska study lies in its optimism. The “Brain Age Gap” is driven by inflammation and waste accumulation—both of which are reversible processes.
The brain is resilient. By implementing the protocols of circadian anchoring, glymphatic optimization (side sleeping), and nervous system regulation, we can halt the acceleration of aging. In 2025, sleep is no longer a passive state of rest; it is the most potent, evidence-based anti-aging intervention available.
The 12-month gap is not a sentence; it is a choice. By prioritizing sleep, we choose to keep our brains younger, sharper, and more resilient for the years to come.









