Is Hidden Inflammation Making Your Organs Age Faster Than Your Actual Age?
The contemporary understanding of human longevity has transitioned from a chronological observation of time to a complex evaluation of biological plasticity and cellular integrity.
In the early 21st century, the identification of “inflammaging”—a state of persistent, low-grade, systemic inflammation—has emerged as the central pillar of geroscience, providing a mechanistic explanation for why individuals of the same chronological age experience vastly different health outcomes.
Emerging research from 2024 and 2025 suggests that this inflammatory burden is not an inevitable byproduct of the human condition but rather a pathological response to the industrialized environment, capable of accelerating the biological age of vital organs by five to ten years relative to the calendar age.
The Industrialization of the Immune System: Evolutionary Mismatch and the Rise of Sterile Inflammation
A foundational premise of modern immunology is that the human immune system evolved over millions of years to provide a rapid, acute response to life-threatening infections and physical trauma.
Industrialized
Obesity, Sedentary, Pollution
Linear Increase with Age
High (CVD, Diabetes, Alzheimer’s)
However, the shift from ancestral, high-pathogen environments to the “sterile” conditions of industrialized society has created an evolutionary mismatch.
Recent cross-cultural studies comparing diverse global populations reveal that inflammaging is not a universal phenomenon but is instead characteristic of industrialized lifestyles.
Comparative Population Analysis of Inflammaging Markers

Data collected from 2024 and 2025 reveal a striking divergence in the inflammatory trajectories of industrialized cohorts compared to non-industrialized indigenous groups. The following table synthesizes the findings from recent analysis of cytokine profiles across these populations.
The lack of an inflammaging signature in groups like the Tsimané, despite their high exposure to infectious agents, suggests that ancestral lifestyles involving extreme physical exertion and high-fiber, low-fat diets may provide a protective buffer against the systemic decay seen in the West.
In industrialized settings, the immune system is constantly triggered by “sterile” stressors—such as visceral adiposity and processed food additives—which do not resolve in the manner of an acute infection.
Instead, these stressors foster a non-resolving inflammatory state that persistently damages tissues and accelerates the functional decline of the heart, brain, and kidneys.
The Role of the Exposome in Biological Aging

The concept of the “exposome” gained prominence in 2025 as the primary driver of biological age acceleration. Unlike the genome, which is relatively fixed, the exposome encompasses the cumulative environmental exposures from conception to death.
Research suggests that approximately 95% of chronic diseases are caused by the exposome’s impact on gene expression rather than the genes themselves.
In industrialized environments, this includes the accumulation of microplastics, pesticides, disruptions to circadian rhythms, and chronic social isolation—all of which act as pro-inflammatory stimuli that “age” the immune system.
Molecular Mechanisms: The Vicious Cycle of Systemic Decay
The acceleration of organ aging by a decade is not merely a metaphor; it is the result of specific molecular hallmarks interacting in a self-perpetuating feedback loop. When chronic inflammation becomes systemic, it activates a series of destructive pathways that impair cellular repair and promote tissue fibrosis.
Cellular Senescence and the Spread of Aging Signals
A central mechanism in this process is the accumulation of senescent cells—cells that have reached their replicative limit and entered a state of irreversible growth arrest.
These cells do not merely occupy space; they actively secrete a cocktail of pro-inflammatory cytokines, chemokines, and proteases known as the senescence-associated secretory phenotype (SASP).
The SASP acts as a localized and systemic source of inflammaging, spreading the aging signal to healthy neighboring cells and inducing secondary senescence in a process often referred to as “bystander effect”.
Mitochondrial Dysfunction and Oxidative Stress

Inflammaging and mitochondrial health are inextricably linked. Persistent inflammatory signaling increases the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) within the cell.
These reactive molecules damage mitochondrial DNA (mtDNA), impairing the organelle’s ability to produce adenosine triphosphate (ATP) efficiently. This energetic failure leaves cells unable to perform critical maintenance and repair functions, leading to the buildup of damaged proteins and genomic instability.
Furthermore, when damaged mitochondria leak mtDNA into the cytosol, it is recognized by the innate immune system as a danger-associated molecular pattern (DAMP), triggering the NLRP3 inflammasome and creating a perpetual cycle of inflammation-driven mitochondrial decay.
The iAge Protocol: Neural Network Quantification of Immune Age
CXCL9
BIOLOGICAL CONSEQUENCE:
Promotes arterial stiffening and T-cell infiltration.
In 2024 and 2025, the ability to measure the "rate of aging" has surpassed the accuracy of traditional blood panels through the development of specialized biological clocks. The most prominent among these is the iAge clock, developed by researchers at Stanford University and the Buck Institute for Research on Aging.
Architectural Foundations of the Inflammatory Clock

The iAge clock was constructed using data from the "Thousand Immunomes Project," a 12-year longitudinal study that analyzed over 10,000 parameters of the human immune system.
The model employs a deep guided autoencoder—a type of neural network designed to find meaningful patterns in highly redundant and noisy biological data.
The calculation of an individual's inflammatory age can be conceptualized through the deviation from the chronological mean, represented as:
Delta Age Inf = iAge Predicted - Age Chronological
A significant positive value for Delta Age Inf indicates accelerated biological aging, while a negative value correlates with superior healthspan and resilience.5 Clinical validation has shown that iAge can predict the development of physical frailty up to seven years before its clinical manifestation.
Critical Proteomic Biomarkers in iAge Analysis
The iAge model identifies a specific subset of proteins that serve as the primary drivers of the aging score. These proteins are not merely markers of age; they are active participants in the deterioration of organ systems.
Validation of this model in centenarians and supercentenarians reveals that these individuals possess an inflammatory age that is, on average, 40 years younger than their chronological age.
In exceptional cases, supercentenarians have been measured with an iAge score 80 years below their calendar years, suggesting that "immortal" immune systems are the secret to extreme human longevity.
suPAR: The Clinical Sentinel of Chronic Stress
Complementary to proteomic clocks is the measurement of soluble urokinase plasminogen activator receptor (suPAR), which has emerged in 2025 as the gold-standard biomarker for evaluating an individual's chronic inflammatory burden. Unlike C-reactive protein (CRP), which acts as an acute-phase reactant and fluctuates rapidly in response to short-term illness or stress, suPAR is remarkably stable in the bloodstream.
Comparative Stability: suPAR vs. CRP

The following table contrasts the behavior of suPAR and traditional inflammatory markers, highlighting why suPAR is a superior predictor of biological aging.
Data indicate that elevated suPAR levels correlate with a biological aging rate that places individuals 10 years ahead of their chronological peers. This acceleration is visible in the phenotype: those with high suPAR scores exhibit an older facial appearance, faster cognitive decline, and reduced physical independence.
As of Spring 2025, the suPAR test is increasingly available for research and personalized health tracking, allowing individuals to monitor the impact of lifestyle interventions on their inflammatory baseline.
The Cost of Acceleration: Organ-Specific Decay
The "10-year acceleration" caused by chronic inflammation is not a uniform systemic decline but a targeted degradation of vital organ systems. When the immune system remains in a state of low-grade activation, it effectively "digests" the body's functional reserves.
Cardiovascular Degradation: From Stiffening to Rupture

In the cardiovascular system, inflammaging acts as a persistent catalyst for endothelial dysfunction and atherosclerosis. Pro-inflammatory cytokines like IL-6 and CRP promote the infiltration of macrophages into the arterial wall, where they consume oxidized cholesterol and transform into foam cells, forming the core of atherosclerotic plaques.
Chronic inflammation also upregulates the production of metalloproteinases, which weaken the fibrous cap of these plaques. When a plaque ruptures, it triggers the acute thrombotic events that kill roughly 25% of the human population through heart attacks and strokes.
Furthermore, specific iAge markers like CXCL9 are directly linked to ventricular remodeling and the loss of arterial elasticity, making the heart of an "inflamed" 50-year-old functionally equivalent to that of a 60-year-old.
Neuroinflammation and the "Microglial Tipping Point"

In the central nervous system, inflammaging manifests as the chronic activation of microglial cells, the brain's resident immune defenders. In a healthy, young brain, microglia act as "gardeners," pruning synapses and clearing debris.
However, as the systemic inflammatory burden increases, these cells reach a tipping point where they become neurotoxic.
This "microglial priming" leads to the overproduction of TNF-alpha, which damages neurons and facilitates the accumulation of beta-amyloid and tau proteins—the pathological hallmarks of Alzheimer's and Parkinson's diseases.
Renal Decline and the Immune-Kidney Feedback Loop

The kidneys are particularly sensitive to systemic chronic inflammation. High levels of suPAR have been clinically linked to the development of chronic kidney disease (CKD) and the progression to end-stage renal failure.
The relationship is bidirectional: systemic inflammation damages the podocytes (specialized filtration cells) in the kidney, while the resulting decline in renal function prevents the clearance of pro-inflammatory cytokines, creating a lethal cycle of multi-organ failure.
Musculoskeletal Erosion: Sarcopenia and Osteoporosis
The impact of inflammaging on muscle and bone is profound. Elevated systemic cytokines interfere with myogenesis and promote the degradation of muscle protein, leading to sarcopenia—the age-related loss of muscle mass and strength.
This process is often exacerbated by "osteosarcopenia," where the same inflammatory signals stimulate osteoclasts (bone-resorbing cells) and inhibit osteoblasts (bone-forming cells), leading to increased bone fragility and risk of fractures.
Recent analysis identifies the protein periostin (POSTN) as a key molecular bridge connecting mechanical stress and inflammation to structural deterioration in the spine and joints.
Transgenerational and Early-Life Programming
The trajectory of biological aging is not solely determined in adulthood; it is often established through transgenerational and early-life environmental factors. The theory of developmental origins suggest that inflammation may start two or three generations before an individual is born.
Maternal Influence and Fetal Programming

If a mother experiences excessive inflammation during pregnancy—due to factors such as obesity, smoking, or chronic psychosocial stress—this signal is transmitted to the fetus.
Children born under these conditions exhibit a "pre-aged" immune system, with a higher risk of developing autism, early-onset diabetes, and cardiovascular diseases.
This suggests that the "10-year" acceleration can be a baseline condition for some individuals, requiring even more aggressive intervention in early life to normalize their aging trajectory.
Childhood Adversity and the Speed of Aging
suPAR
Cumulative immune activation/burden
5 to 10-year acceleration potential
Predicting CVD, CKD, and mortality
Longitudinal studies, such as the Dunedin Multidisciplinary Health and Development Study, have used clocks like DunedinPACE to show that exposure to violence, poverty, or neglect in childhood significantly increases the "pace" of biological aging.
These individuals show physiological signs of aging—such as reduced lung function, cognitive decline, and increased suPAR levels—decades earlier than those with stable childhoods.
The Space-Aging Paradox: Insights from Microgravity

Space travel has provided a unique laboratory for studying accelerated aging. Astronauts, who are among the healthiest humans on Earth, experience a rapid acceleration of biological aging due to the stressors of microgravity and high-energy radiation.
During spaceflight, individuals exhibit many of the same physiological changes seen in the elderly: loss of bone density, muscle atrophy, and a significant spike in systemic inflammatory markers.
Studying the "immune-aging" of astronauts has allowed scientists to identify specific proteins and pathways that can be targeted on Earth to mitigate age-related decline.
Therapeutic Frontiers: Reversing the Biological Clock
As the evidence linking chronic inflammation to accelerated aging has solidified, the focus of geroscience has shifted toward interventions that can "reset" the biological clock. In 2025, three primary domains of intervention have emerged: bioelectronic medicine, nutritional geroscience, and pharmacological senolytics.
Bioelectronic Medicine: Vagus Nerve Stimulation
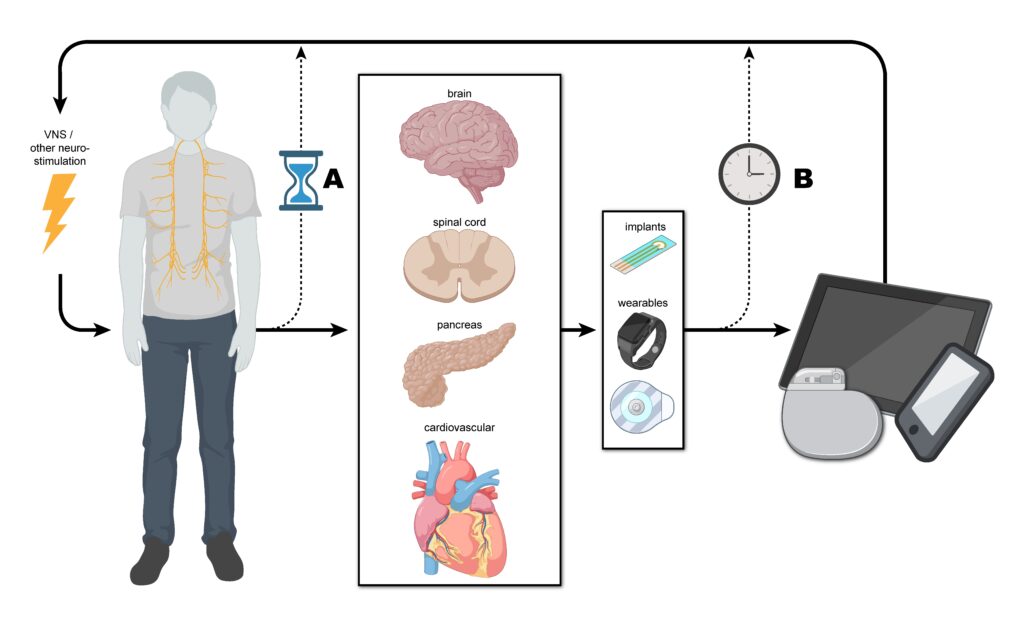
The discovery of the "inflammatory reflex" by Dr. Kevin Tracey and his colleagues has revolutionized the treatment of chronic inflammation. The vagus nerve serves as the body's primary neural pathway for regulating the immune response.
By stimulating the vagus nerve—using either surgical implants or non-invasive external devices—clinicians can trigger the release of neurotransmitters that tell the spleen and other immune organs to stop producing pro-inflammatory cytokines.
This "electroceutical" approach has shown success in clinical trials for rheumatoid arthritis, inflammatory bowel disease, and heart failure. Research indicates that regular vagus nerve stimulation (VNS) could potentially add decades to a healthy lifespan by keeping systemic inflammation within a youthful range.
Nutritional Geroscience: The Longevity Diet and FMD

The role of nutrition in modulating inflammaging has moved beyond general advice to precise protocols. Dr. Valter Longo’s "Longo Diet" and the "Fasting-Mimicking Diet" (FMD) have demonstrated the ability to slow biological aging at the molecular level.
A critical insight from the 2024 DO-HEALTH trial is that the combination of Vitamin D3, Omega-3 fatty acids, and regular exercise can reduce biological age by 2.9 to 3.8 months over three years, effectively giving the body an "extra season" of youth for every three years of chronological time.
Pharmacological and Supplemental Interventions

The identification of "senolytics"—compounds that selectively target and kill senescent cells—represents a major pharmacological leap.
In addition to pharmaceutical candidates, several naturally occurring molecules have been validated in 2025 for their anti-inflammatory and longevity-promoting properties.
Urolithin A: A gut metabolite that induces mitophagy (the clearance of damaged mitochondria) and has been shown to reduce both senescence and systemic inflammation.
NAD+ Boosters (NMN/NR): These precursors increase levels of Nicotinamide Adenine Dinucleotide (NAD+), which is essential for the function of sirtuins—proteins that repair DNA and regulate inflammatory genes.
EGCG, Resveratrol, and Curcumin: These polyphenols exhibit potent anti-inflammatory effects by inhibiting NF-kappaB activation and scavenging free radicals.
Spermidine and Sulforaphane: Compounds found in wheat germ and cruciferous vegetables that stimulate autophagy and bolster the body's antioxidant defenses.
Socioeconomic and Economic Implications of Biological Divergence

The realization that chronic inflammation can age organs 10 years faster has massive implications for global economies. In the United States alone, the top 10 conditions affecting individuals over 65—including high blood pressure, obesity, and arthritis—are all closely linked to chronic inflammation.
Economists estimate that extending the healthy human lifespan by just one year would save $37 trillion over a decade by reducing healthcare costs and increasing the productive years of the population.
The development of "at-home" diagnostics, such as saliva-based DNA methylation tests or AI-driven facial morphometry ("Healthy Selfies"), could democratize biological age assessment and allow for mass-scale preventative interventions.
Synthesizing the 10-Year Acceleration Phenomenon

The collective body of research from 2024 and 2025 confirms that chronic inflammation is the primary driver of the "aging gap." Individuals with high inflammatory burdens—whether due to early-life adversity, modern lifestyles, or genetic predisposition—experience a rate of organ decay that significantly outpaces their chronological years.
However, the findings also offer a message of profound biological plasticity. The fact that non-industrialized populations do not show inflammaging, and that clinical interventions like VNS and FMD can reverse biological age markers, suggests that the "10-year acceleration" is not a fixed destiny.
By shifting the medical paradigm toward the early detection and systematic suppression of chronic inflammation, it is possible to synchronize biological time with chronological time—or even to achieve a decelerated aging state where the body remains functionally young well into its second century.
Strategic Recommendations for Life-Extension Practice
For medical professionals and individuals focused on healthspan optimization, the data suggest a multi-layered approach to managing the inflammatory axis.
Baseline Quantification: Utilize stable biomarkers like suPAR and proteomic clocks like iAge to establish a biological age baseline. A suPAR score above 6.0 ng/mL should be viewed as a clinical indicator of accelerated aging requiring immediate lifestyle or therapeutic intervention.
Exposome Mitigation: Reduce "sterile" inflammatory triggers by minimizing exposure to environmental toxins, improving sleep hygiene, and managing chronic stress through mindfulness or biofeedback.
Metabolic Resetting: Implement periodic cycles of Fasting-Mimicking Diets (FMD) to clear senescent cells and stimulate stem cell-driven tissue regeneration.
Targeted Supplementation: Integrate high-dose Omega-3s, Vitamin D3, and mitochondrial support agents like Urolithin A and NAD+ boosters into daily routines, especially for individuals over the age of 40.
Neural Modulation: Explore vagus nerve stimulation (VNS) technologies as a means of non-pharmacological immune regulation.
As the field of geroscience moves toward the year 2026 and beyond, the ability to manipulate the "inflammatory crystal ball" will likely define the next great leap in human health, potentially adding decades of vibrant, disease-free life to the global population.